Part 1: Setting the stage
Last time we dipped our toes into the neutron, particularly how it interacts with matter. This time we’re going to continue that discussion a bit further into the realm of nuclear fission. We’ll see how fission works, broadly and why some isotopes can cause the UN Security Council to blow a gasket. Let us begin with our old friend uranium. Uranium ore, the stuff you pull out of the ground is about 99.3% uranium-238 with the remaining 0.07% being uranium-235. The reason for this discrepancy is due to the different half-lives of the two isotopes. They, like all the heavy elements on Earth were formed in stellar supernovas eons ago (and we’ll have more to say on that when we get to the H-Bomb) but 235 has a half-life of 7.03E8 years where as 238 has a half-life of 4.47E9 years. Incidentally four and a half billion years is about the age of the Earth. As a result there is far less 235 available in nature but they both exist together in natural ores.
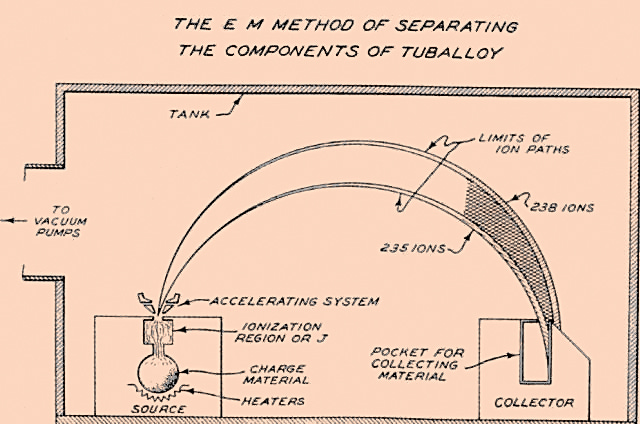
In the early 1930’s when the neutron was first discovered the existence of 235 wasn’t yet known. That would come in 1935 when an Canadian-American physicist, Arthur J. Dempsey isolated a small sample of the metal using a technique called mass spectroscopy. By using an electromagnetic field it is possible to separate isotopes by their mass. This isotope separation is called a mass spectrum. To keep a very long story short, you ionize the material you’re looking at and then accelerate the ions using electric fields into a vacuum chamber. The vacuum chamber is stuck between the poles of a magnet and so the magnetic fields deflect the beam in an arc (charged particles are deflected into circular paths when exposed to magnetic fields). As different isotopes have different masses, the amount of deflection is different for each isotope; the lighter ions follow a tighter curve while the heavier ones follow a larger curve due to inertia. This deflection spreads out the isotopes into a ‘spectrum’ from low to high mass, hence the name. There will be much more to say on this when we discuss enrichment and particle accelerators as the same principle of magnetic deflection is exploited by cyclo- and synchrotrons.
When 235 was discovered it was greeted with interest but the ramifications of what it might mean did not become apparent until 1939. In the meanwhile, Enrico Fermi’s laboratory in Rome began blasting elements with neutrons to see what would happen. I say blasting but really they were taking a small neutron source of radon and beryllium and letting it shoot neutrons at targets on a tabletop. They were building off the work of Chadwick who had discovered neutrons and Curie the Younger who had won the 1935 Nobel Prize for artificially induced radioactivity through alpha particle bombardment. By bombarding stable aluminum with an alpha particle, Curie and her husband found that a radioactive isotope of phosphorus with a half life of two and a half minutes was formed.
This was inspiration to Fermi and his co-conspirators in Rome who figured they could find neat radio-isotopes through neutron driven reactions. Unlike alpha particles, neutrons don’t have to worry about overcoming the coulomb barrier to hit the nucleus. Using radon-beryllium1 and radium-beryllium neutron sources, they worked their way up through the periodic table and made a very interesting discovery along the way. In their laboratory experiments were typically conducted on a marble top table where the source and sample would be placed with the relevant radiation detectors; typically a Geiger counter to detect the activated sample material. From the induced radiation level the reaction rate could be estimated. One day though the experiments were being conducted on a wooden table instead of the usual marble and a marked increase in the reaction rate was noted. At first the team assumed the problem lay with their detectors but that was ruled out. Eventually it was realized that the differences in table composition was the culprit. Marble contains calcium (A~43; there are several stable isotopes) which is a much larger nucleus than the neutron. As such when neutrons collide with it, the neutron simply scatters in an inelastic collision, losing very little of its initial energy. A wooden table by contrast contains a lot of hydrogen and so neutrons hitting hydrogen nuclei (which are essentially the same mass as the neutron) undergo inelastic collisions wherein the neutron loses a lot of energy to the hydrogen nucleus, slowing it down.
This is called moderation and it can be modeled pretty simply. We start with an initial neutron energy, let’s say 3.6 MeV as this was the average neutron energy of the Ra-Be neutron sources Fermi and his team were using. We want to see how many collisions it will take to slow the neutron to thermal energies at about 0.025 eV2.
The ratio between the average initial and final kinetic energy of a neutron that hits a nucleus is given by:
Where N is the number of collisions (so for one collision N=1) and ε is a logarithmic constant of energy decrease. It’s defined by the following3:
Where A is the atomic mass number of the target nucleus. So to illustrate this, let’s see how many collisions it will take for a neutron at 3.6 MeV to become thermalized in wood and marble. To keep things simple we’ll just look at hydrogen (A=1; a major ingredient in wood), and calcium (A=40; a major ingredient in marble). With hydrogen ξ =1.
Then solve for the number of collisions.
Repeating the process with calcium first we find ε.
Then we solve for the number of collisions.
Thanks to inelastic collisions, it takes a lot more knocks to thermalize a neutron in a calcium rich material than in hydrogen.
As we saw last time, interaction cross sections tend to increase as neutron energy decreases. Some neutrons flew into the table, were slowed by the hydrogen and then were ricocheted out to the target but with much less energy increasing the likelihood they would interact with a target nucleus. This effect was definitively proved by placing a block of paraffin wax (which has a very high hydrogen content) in between the neutron source and sample causing a massive increase in reaction rate. Uranium was one element which really exhibited this effect and from decay signatures detected with Geiger counters Fermi and his group thought they had found new transuranic elements. Transuranic meaning elements beyond uranium, Z=92. They were but they did not know they were also creating elements much lower down the periodic table. This work with elements led to Fermi getting the Nobel Prize in 1938.
In 1937 the Curies reported finding a uranium bombardment product which had a half life of 3.5 hours and bizarrely seemed to have the chemical properties of lanthanum. Given that uranium is element 92 and lanthanum element 57 there was no known mechanism to produce that result. It was assumed that the element was the chemically similar actinium as it is much closer to uranium on the periodic table or a hypothetical transuranic. In either case the nuclear reactions involved would be unorthodox. The Curies didn’t know it but they actually were detecting a radioactive isotope of lanthanum.
Part 2: The Road to Fission
In December of 1939 Fritz Strassman, working under Otto Hahn at the Kaiser Wilhelm Institute in Berlin performed the experiment that really gets our story going. Strassman was a chemist brought in to be part of a team that included Hahn (also a chemist) and Hahn’s long time collaborator, the physicist Lise Meitner. As a team they had been investigating neutron capture by uranium nuclei, to better understand transuranic processes. By 1938 though Meitner was corresponding to the other two team members by letter from Sweden; under Hitler her Jewish heritage had been used to force her out of German academia.
Due to the team’s chemical background, it was decided to try and separate out transuranic elements from bombarded uranium samples. At that point, the electron orbital structure of atoms was not as well understood and the actinides4 were not considered a separate category like the lanthanides as they are today with electrons filling up the f-shell after the outer electron orbitals are filled. Instead, they were treated as seventh row elements with actinium (89) sitting under lanthanum (57), thorium (90) under hafnium (72), protactinium (91) under tantalum (73) and uranium (92) under tungsten (74). As such, hypothetical transuranic elements were refereed to by adding the prefix ‘eka-’ (from a greek word meaning next or beyond) to the front of the element above. So element 93 was called ‘eka-rhenium’ and element 94 was called ‘eka-osmium’ and so on.
It was assumed that these new elements would have similar chemical properties to the elements above them. It was also assumed that any radioisotopes produced following the neutron bombardment of uranium must be transuranics. The problem was that nine apparent elements with different half lives were detected. Far more than Fermi’s team had seen. These were initially taken to be transuranic elements created via complicated beta and even alpha decay chains though the physicality of these chains were heretofore unknown. This was not entirely a problem, as many bizarre effects had been found in nuclear research up to this point though it was pushing the envelope. The real problem was the chemistry.
As was the case with the Curies in 1937, the team at the Kaiser Wilhelm Institute began to identify something strange. Strassman had found incontrovertible evidence of barium in their reaction products. Barium is element 56, impossibly far from uranium via any alpha or beta decay. Writing to Meitner in Sweden they asked their collaborator what, if any mechanism could account for the creation of barium from a single incident neutron colliding with a uranium nucleus. What she and her nephew, the physicist Otto Frisch (who was visiting his aunt for the holiday season) came up while out on a walk in the snow was nuclear fission.
To understand what they did, we’ll start with the liquid drop we first saw back in episode 4. As you may remember, you can model the nucleus as being analogous to a drop of liquid. Meitner and Frisch reasoned that a neutron introduced into an isotope would cause it to rapidly become unstable, elongating to the point that the columbic repulsion of the protons would push the nucleus apart wherein it would form two smaller nuclei. Remember, the strong force has a very short range, and so if the nucleus was disturbed into an elongated shape the strong force would not hold the particles on the ends of the shape together any longer. Instead, the electromagnetic repulsion of the protons (which has a much larger range) would overpower the diminished strong force and rip the nucleus in two. In their paper to Nature in 1939 it was estimated that the fission process (a term Frisch borrowed from the process of cellular fission in biology) would give off approximately 200 MeV of energy per event. The reaction found by Strassman was as follows; with the caveat that the neutron emission was only suspected at the time.
Let’s assume that the nucleus has disassociated into two spheres, one of krypton and the other of barium. They’re sitting right next to each other. First we find the radii of the two spheres which will give us the radius of separation and then find the columbic force acting on them. From that we can find the energy of the reaction.
So starting from our old nuclear radius formula5 we find values of the radii for barium and krypton.
By adding these together we find the total radius to be considered.
To find the coloumb potential or the potential energy between two electrical charges we simply integrate the the function for electrostatic force.
As a reminder k is the coloumb constant of 8.987E9 Newton-meters per Coloumb squared. q1 and q2 are the charges of the two particles under consideration; in this case the barium and krypton nuclei. Remember when using either of these functions, the q value needs to be in coulombs, so for example the q value for barium would be 56 (the number of protons in a barium nucleus) times the elementary charge, 1.602E-16 C.
Entering in the relevant values we find the potential energy between the two nuclei.
This is around our assumed 200 MeV value for fission so things are beginning to make sense. Not bad for an extremely rough calculation.
In January of 1939, shortly after finishing his work with his aunt, Otto Frisch ventured to Copenhagen where he was working as a researcher and discussed the experimental and theoretical work on fission with his friend Niels Bohr, the famed Danish theoretical physicist. Bohr was scheduled to take a visit to the United States as a guest lecturer at Princeton for a semester and brought the news of fission with him to the new world.
News of the fission process had greatly excited the physics community (and it was a community back then, this was just before the shift to the modern Big Science era) in both Europe and America as the results from the Hahn, Strassman, Meitner, Frisch collaboration made their way to America first through Bohr and then through papers published. A crucial series of experiments determined that fast neutrons6 induced fission in uranium and thorium but thermal neutrons only seemed to cause fission in uranium. Bohr realized something important regarding the three isotopes. Parity arguments could explain the discrepancy. We have seen parity discussed before in the semi-empirical mass formula for nuclei7. The final term in the function, Ω8 is the paring term and it is determined by the even or odd numbers of nuclei in the nucleus.
There are three states for Ω; If A and Z are both even numbers, then the binding energy increases Ω. If A is an even number and Z is an odd number then the binding energy decreases as the odd proton is unpaired. If A is odd then Ω is zero. Knowing this, Bohr was able to find an explanation for the differences in neutron energy that enabled fission in thorium and uranium.
Thorium (element 90) exists as essentially a mono-isotope in nature as thorium-232. Thorium has 90 protons and 142 neutrons, both are even numbers and so by the paring term, the paring energy term is maximized. Uranium on the other hand has two isotopes in nature, uranium-238 and the rarer uranium-235. Uranium-238 has 92 protons and 146 neutrons, again satisfying the requirement for maximum paring energy. Uranium-235 by contrast again has 92 protons but only 143 neutrons. Meaning that A is odd and so the paring term is 0. Meaning it has slightly less binding energy than uranium-238 or thorium-232. From this Bohr reasoned that the increase in binding energy meant that thorium-232 and uranium-238 needed a higher energy neutron to disturb them enough to undergo fission, while the more shaky uranium-235 was apt to go to pieces at any neutron energy. Building on this realization Bohr worked with the American physicist John Wheeler at Princeton to build a model of the fission process. It’s a bit tricky, but I’m going to do my best to cover it for you so please use the bathroom, fix yourself a stiff drink and come back when you’re ready.
Part 3: Bohr-Wheeler Theory
So, where does the energy come from to induce fission? It comes from either the incident neutron or the alteration in binding energy caused when the nucleus absorbs the neutron and becomes a new nucleus. That second term is a bit tricky to understand so let’s unpack it for a moment. When a nucleus captures a neutron, a series of internal changes occurs as the nucleons get shuffled around into new nuclear orbitals. The details of this will be covered later in my long awaited post on quantum mechanics and the nucleus but for now understand that when a neutron is captured a new nucleus is formed even if only for an instant.
What Bohr and Wheeler found was that there is a limit, the spontaneous fission limit beyond which any nuclei of sufficient size would immediately tear itself apart at the slightest perturbation. This phenomenon of spontaneous fission will play a huge role in bomb design as we will see but for now let’s consider a nucleus as a liquid drop and consider how this limit is derived. We have our nucleus, and it’s just absorbed a neutron, setting up a perturbation. Now what happens when we deform the nucleus? The number of nucleons stays constant, the volume stays constant, the paring and asymmetry terms stay the same. All that changes when a nucleus is disturbed is the surface area term and the columbic forces as the protons move around.
Start with our old friend the semi-empirical mass formula.
The terms corresponding to the surface area and columbic forces are:
The nucleus starts as a sphere of charge and the electrostatic potential energy of a sphere of charge is defined like so9.
The surface area of a sphere is this certified geometry classic.
As the energy of the incident neutron spreads through the sphere, an oscillation of the sphere is set up causing the sphere to become more elongated, wiggling back and forth. As this happens the surface area of the one time sphere begins to increase as it wobbles into an ellipse shape.
Using a power series we can model this increase in surface area as a function of the eccentricity, ϵ. Eccentricity is a math term that means how elongated an ellipsoid is. The greater the eccentricity, the longer and thinner the ellipsoid. A sphere has an eccentricity of 0. An ellipsoid has an eccentricity between 0 and 1.
Since the surface area term here is proportional to the surface energy term we can define the surface energy term using our expansion.
We can also likewise take a series expansion of the columbic force. You will notice a pattern, that our functions consist of the initial energy value plus or minus a factor determined by the eccentricity of the deformed nucleus.
And as before we can replace the potential term with our coloumb energy term. Because we’re dealing with high Z elements here, we can simplify the second term.
As the deformation increases, the surface energy increases, meaning the surface tension wants to pull the nucleus back into a sphere. By contrast, as the nucleus stretches out further and further the electrostatic energy starts to want to pull the nucleus apart, and since in this notation, returning to sphere is positive, and continued elongation is negative, the electrostatic term is defined as negative because it wants to pull the nucleus away from spherical existence. So to determine the net energy change in the nucleus due to the distortion we just take the two terms that are defined by the eccentricity since they are what actually changes. With a little algebra we get this.
As we defined the contraction driven by the surface tension as positive, if the term within the brackets becomes negative the nucleus will not be stable and the electric forces will pull the nucleus out longer and longer until it snaps into two. At that point electrostatic forces push the two new nuclei away and we’re back to what Meitner and Frisch first proposed.
So if the bracketed term remains positive the nucleus remains stable and we can find that limit of stability. To do this, we simply set the bracketed terms equal to each other and solve.
After a little algebra we find the following.
At this point, the nucleus can spontaneously undergo fission. This is called, unimaginatively the Z2/A limit for fission. With the correct constants from the semi-empirical mass equation we find the limit is around 45.
Looking at uranium; with 238 we see that it has a ratio of 35.6, well below the spontaneous fission threshold. Similarly with 235 we see a ratio of 36. This means that an activation energy has to be exceeded to induce fission. What that energy is depends on the isotope in question and to calculate it is again beyond this shitpost.
Now let’s assume they both capture a thermal neutron. A thermal neutron is important here because it essentially has no additional energy. The energy in the system is caused by the incident nucleus changing into a new one with a new nucleon arrangement.
Now what you’re looking at there is something called a compound nucleus. It is formed between the nucleus and the incident neutron for a very short period before some form of reaction occurs. We’re talking 10E-12 ~ 10E-13 seconds. But for fission that instant of compound nucleus formation is the critical to the process. The Q value or energy created in a given reaction is the key. Energy created in this reaction is contained in the nucleus, causing it to wobble. If it is greater than the fission threshold, then the nucleus splits. Looking at our uranium isotopes we have two fission thresholds.
These are the values for the compound nuclei which are created by the neutron bombardment of 238 or 235.
Running the energy calculations for uranium-238 we find a Q value of 4.8 MeV. This is -1.65 MeV below our threshold value of 6.45 MeV. No fission, just a wobble and a capture event that can eventually make plutonium. Remember, this is a thermal neutron, so no additional energy comes from kinetic energy. Thermal neutrons have a kinetic energy around 0.025eV, so essentially nothing. The energy imparted to the nucleus is caused by nuclear forces due to alterations in binding energy. With uranium-235 we find a Q value of 6.5 MeV giving us +0.83 MeV over the threshold for fission. No matter the energy of an incident neutron then, uranium-235 can fission. Uranium-238 can fission as well but only from interacting with a fast neutron with an energy of at least 1.65 MeV to make up the difference between the Q value and the threshold. This fast fission effect played a role in the worst nuclear accident in US history as we shall see down the road.
Anyway that was quite a bit. I could go on but I think the point has been made. Next time will either be a discussion on the chain reaction and reactors (with some statistical thermodynamics) or a discussion on quantum mechanics.
Books I would recommend for this section have been mentioned before, namely:
Nuclear Radiation Physics Third Edition by Lapp and Andrews, 1963 Prentice-Hall; Specifically chapter 13 which discusses fission.
The Physics of the Manhattan Project by Reed, 2011 Springer; Specifically chapter 1: pages 20-36 which discusses Bohr-Wheeler theory, the fission barrier and associated derivations. Also many useful tables of relevant figures, I made use of them here.
The Making of the Atomic Bomb, Rhodes, 1987 Simon and Schuster; Very helpful for historical context.
In addition there are lots of fun papers to read if you really want to dig in.
Possible Production of Elements of Atomic Number Higher than 92, Fermi, 1934, Nature
Neutron Capture and Nuclear Constitution, Bohr, 1936, Nature
On the Absorption and the Diffusion of Slow Neutrons, Amaldi, Fermi, 1936, Nature
Disintegration of Uranium by Neutrons: a New Type of Nuclear Reaction, Meitner, Frisch, 1939, Nature
The Mechanism of Nuclear Fission, Bohr, Wheeler, 1939, Physical Review
Some Fission Products of Uranium, Segre, Wu, 1940, Nature
Radon-beryllium was used as the radioactive radon gas produced in radium decay was also an alpha emitter and could be used to produce neutrons via alpha bombardment of beryllium.
0.025 eV neutrons are called thermal because they have approximately the same energy as room temperature molecules. From thermodynamics we know the average kinetic energy of a given particle at a certain temperature is:
The term under the square root is the average velocity of a particle of mass m at temperature (in kelvin) T; k is the Boltzmann constant (1.831E-23 J/K). The mass in this case is the mass of the neutron (1.675E-27 kg) and the temperature is 298K or room temperature in scientific circles. Room temperature is considered an important threshold as a neutron cannot lose more energy on average than the average energy of the nuclei of the molecules with which it is interacting. Running the numbers gives:
Named after the leading element of the series, actinium; element-89
Fast neutrons have a high kinetic energy, with a range from ~10 keV to ~10 MeV. Typically 1-2 MeV is what is considered fast but it really depends on the context. Most neutrons created in nuclear reactions begin life as fast neutrons.
A good explanation of this can be found in the Feynman Lectures on Physics; Volume II, chapter 8. Also this is a decent explanation.




